ONE OF THE MOST COMMON AND MOST VERSATILE SOURCES OF DC IS THE CELL.
The term cell means self-contained compartment, and it can refer
to any of various different things in (and out of) science. In electricity and
electronics, a cell is a unit source of dc energy. There are dozens of
different types of electrical cells.
When two or more cells are connected in series, the result is known as a battery.
Kinetic and potential energy .
Energy can exist in either of two main forms. Kinetic energy is the
kind you probably think of right away when you imagine energy. A person
running, a car moving down a freeway, a speeding aircraft, a chamber of
superheated gas—all these things are visible manifestations of
kinetic energy, or energy in action. The dissipation of electrical power, over
time, is a form of kinetic energy too.
Potential energy is not as
vividly apparent. When you raise a block of concrete into
the air, you are creating potential energy. You remember the units called
foot pounds, the best way to measure such energy, from school physics
classes. If you raise a one-pound weight a foot, it gains one foot pound
of potential energy. If you raise it 100 feet, it gains 100 foot pounds.
If you raise a 100-pound weight 100 feet, it will gain 100 × 100, or
10,000, foot pounds of potential energy. This energy becomes spectacularly evident
if you happen to drop a 100-pound weight from a tenth-story window.
Electrochemical energy
In electricity, one important form of potential energy exists in the
atoms and molecules of some chemicals under special conditions. Early in the
history of electrical science, laboratory physicists found that when metals
came into contact with certain chemical solutions, voltages appeared between the
pieces of metal. These were the first electrochemical cells.
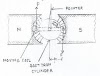
A piece of lead and a piece of
lead dioxide immersed in an acid solution (Fig.)will show a persistent voltage.
This can be detected by connecting a galvanometer between the pieces of metal.
A resistor of about 1,000 ohms should always be used in series with the
galvanometer in experiments of this kind; connecting the galvanometer directly
will cause too much current to flow, possibly damaging the galvanometer and causing
the acid to “boil.”
The chemicals and the metal have an inherent ability to produce a
constant exchange of charge carriers. If the galvanometer and resistor are left
hooked up between the two pieces of metal for a long time, the current will
gradually decrease, and the electrodes will become coated. The acid will
change, also. The chemical energy, a form of potential energy in the
acid, will run out. All of the potential energy in the acid will have been
turned into kinetic electrical energy as current in the wire and galvanometer.
In turn, this current will have heated the resistor (another form of kinetic
energy), and escaped into the air and into space.
Cells and batteries
 Reviewed by Sikha
on
June 19, 2016
Rating:
Reviewed by Sikha
on
June 19, 2016
Rating:
 Reviewed by Sikha
on
June 19, 2016
Rating:
Reviewed by Sikha
on
June 19, 2016
Rating:




No comments:
Post a Comment