Primary and secondary cells
Some electrical cells, once their potential (chemical) energy has all been changed to electricity and used up, must be thrown away. They are no good anymore. These arecalled primary cells.
Other kinds of cells, like the lead-and-acid unit depicted above, can get
their chemical
energy back again. Such a cell is a secondary cell.
Primary cells include the ones you usually put in a flashlight, in a
transistor radio,
and in various other consumer devices. They use dry electrolyte pastes
along with
metal electrodes. They go by names such as dry cell, zinc-carbon cell,
alkaline cell,
and others. Go into a department store and find the panel of batteries,
and you’ll see
various sizes and types of primary cells, such as AAA batteries, D
batteries, camera batteries, and watch batteries. You should know by now that these things are cells,
not truebatteries. This is a good example of a misnomer that has gotten so
widespread that
store clerks might look at you funny if you ask for a couple of cells.
You’ll also see real
batteries, such as the little 9-V transistor batteries and the large 6-V
lantern batteries.
Secondary cells can also be found increasingly in consumer stores. Nickel-cadmium
(Ni-Cd or NICAD) cells are
probably the most common. They’re available in
some of the same sizes as nonrechargeable dry cells. The most common
sizes are AA, C,and D. These cost several times as much as ordinary dry cells, and a
charging unit also costs a few dollars. But if you take care of them, these rechargeable
cells can be usedhundreds of times and will pay for themselves several times over if you
use a lot of “batteries” in your everyday life.
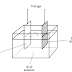
The battery in your car is made from secondary cells connected in series.
These cells
recharge from the alternator or from an outside charging unit. This
battery has cells likethe one in Fig.
. It is extremely dangerous to short-circuit the
terminals of such a battery,
because the acid (sulfuric acid) can “boil” out and burn your skin and eyes.
An important note is worth making here: Never short-circuit any
cell or battery, because
it might burst or explode.
| Lead acid battery |
Primary and secondary cells
 Reviewed by Sikha
on
June 19, 2016
Rating:
Reviewed by Sikha
on
June 19, 2016
Rating:
 Reviewed by Sikha
on
June 19, 2016
Rating:
Reviewed by Sikha
on
June 19, 2016
Rating:





No comments:
Post a Comment